System Used:
M-Series MRI

 Tumor hypoxia and aerobic glycolysis are well-known resistance factors for anticancer therapies. Most tumour cells are subject to conditions in which oxygen is not readily available, known as tumour hypoxia, due to abnormal vasculature. Effective radiotherapy relies on the creation of reactive oxygen species, therefore hypoxic tumors are radiation resistant. Further, both chemotherapy and immunotherapy have been shown to be less effective when exposed to hypoxic environments. Hypoxia also promotes aerobic glycolysis, a metabolic state in which tumours preferentially utilize glucose over oxygen as an energy substrate. In this metabolic state, treatment efficacy is again reduced, and tumour recurrence is promoted. Because of the recognition of hypoxia as an important barrier to cancer treatment, much research has focused on how to overcome or reverse tumor hypoxia.
Tumor-associated macrophages (TAMs) are immune cells recruited to tumors and have been extensively reported for their role in promoting tumour growth and creating an immunosuppressed tumour microenvironment. While TAMs have been shown to produce various factors for tumor survival and inhibit antitumor immune responses, they also provide multiple targets of immunotherapies for limiting tumor progression. Thus, a thorough understanding of the mechanisms by which TAMs promote tumour cell growth provides valuable information which may be used for further novel tumour treatment techniques.
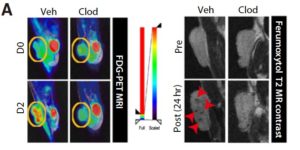
In a recent paper entitled “Tumor-Associated Macrophages Enhance Tumor Hypoxia and Aerobic Glycolysis” researchers from the Seoul National Research Hospital further our knowledge of the role of TAMs in tumour growth and progression by demonstrating that they enhance tumor hypoxia and aerobic glycolysis in mice subcutaneous tumors and in patients with non–small cell lung cancer (NSCLC). Through positron emission tomography (PET) and CD68 TAM immunostaining, a significant correlation was shown between 18fluoro-deoxyglucose (FDG) uptake and CD68 positive TAM showing a link between TAM infiltration and glycolysis in patients with NSCLC. TAM effect on tumour glycolysis was then determined in mice using a Lewis Lung Carcinoma (LLC) tumour model. After LLC tumour injection, TAM depletion was induced using intraperitoneal injections of clodronate (CLOD). Images obtained using the Aspect M7 preclinical MRI and Brightonix SimPET insert, showed that CLOD injections decreased FDG uptake and thus indicated decreased aerobic glycolysis. Further, it was shown that TAM depletion led to a significant increase in programmed death ligand 1 (PD-L1) expression and T-cell infiltration in tumors suggesting that TAMs can significantly modulate tumor metabolism and hinder the efficacy of anticancer therapies.
For more information about the Aspect Preclinical MRI scanners with SimPET insert please contact Scintica Instrumentation.
Tumor hypoxia and aerobic glycolysis are well-known resistance factors for anticancer therapies. Most tumour cells are subject to conditions in which oxygen is not readily available, known as tumour hypoxia, due to abnormal vasculature. Effective radiotherapy relies on the creation of reactive oxygen species, therefore hypoxic tumors are radiation resistant. Further, both chemotherapy and immunotherapy have been shown to be less effective when exposed to hypoxic environments. Hypoxia also promotes aerobic glycolysis, a metabolic state in which tumours preferentially utilize glucose over oxygen as an energy substrate. In this metabolic state, treatment efficacy is again reduced, and tumour recurrence is promoted. Because of the recognition of hypoxia as an important barrier to cancer treatment, much research has focused on how to overcome or reverse tumor hypoxia.
Tumor-associated macrophages (TAMs) are immune cells recruited to tumors and have been extensively reported for their role in promoting tumour growth and creating an immunosuppressed tumour microenvironment. While TAMs have been shown to produce various factors for tumor survival and inhibit antitumor immune responses, they also provide multiple targets of immunotherapies for limiting tumor progression. Thus, a thorough understanding of the mechanisms by which TAMs promote tumour cell growth provides valuable information which may be used for further novel tumour treatment techniques.
In a recent paper entitled “Tumor-Associated Macrophages Enhance Tumor Hypoxia and Aerobic Glycolysis” researchers from the Seoul National Research Hospital further our knowledge of the role of TAMs in tumour growth and progression by demonstrating that they enhance tumor hypoxia and aerobic glycolysis in mice subcutaneous tumors and in patients with non–small cell lung cancer (NSCLC). Through positron emission tomography (PET) and CD68 TAM immunostaining, a significant correlation was shown between 18fluoro-deoxyglucose (FDG) uptake and CD68 positive TAM showing a link between TAM infiltration and glycolysis in patients with NSCLC. TAM effect on tumour glycolysis was then determined in mice using a Lewis Lung Carcinoma (LLC) tumour model. After LLC tumour injection, TAM depletion was induced using intraperitoneal injections of clodronate (CLOD). Images obtained using the Aspect M7 preclinical MRI and Brightonix SimPET insert, showed that CLOD injections decreased FDG uptake and thus indicated decreased aerobic glycolysis. Further, it was shown that TAM depletion led to a significant increase in programmed death ligand 1 (PD-L1) expression and T-cell infiltration in tumors suggesting that TAMs can significantly modulate tumor metabolism and hinder the efficacy of anticancer therapies.
For more information about the Aspect Preclinical MRI scanners with SimPET insert please contact Scintica Instrumentation. 