System Used:
NGB-R

The world of clinical and preclinical research continually breaks barriers, striving to develop innovative methodologies to explore and understand the biological pathways, and disease processes which are necessary to ensure the proper function of the human body and all of its intricacies. Among the most ground-breaking advancements in recent years is the emergence of 3D bioprinting technology and organ/tissue-on-a-chip technology, offering unparalleled opportunities to generate life-like tissues for in vitro studies.
In a recent peer-reviewed publication from a group in the Netherlands and Scotland, Rabussier et al.1 describe the development of an in vitro tissue model of the placental barrier – one of the most poorly studied organs or tissues in the human body; yet one that causes a very high level of morbidity and mortality in the maternal and perinatal setting. The team created this tissue model as a means of studying the properties of the placental barrier to begin to understand the biological mechanisms associated with preeclampsia and other pathologies associated with the placenta and the association with hypoxic and ischemic conditions.
Preeclampsia, a gestational disorder characterized by hypertension and damage to other organ systems, has been the subject of intense medical research due to its significant health implications for both mother and child. Despite progress, the pathophysiology of preeclampsia remains partially understood, as there is an obvious lack of suitable in vivo and in vitro models to help scientists study the complex disease mechanisms. Recent strides in the field have underscored the role of hypoxia, a condition of reduced oxygen availability, in preeclampsia pathogenesis. However, the exploration of hypoxia’s effects in uteroplacental tissues is limited by the inaccessibility of these tissues for in vivo study.
In vitro studies have filled this gap, enabling a closer examination of uteroplacental tissues under hypoxic conditions, however until recently the complexity of the placental barrier has been challenging to replicate in vitro. This group created a protocol allowing them to create a multi-layer tissue that they studied and found closely resembled the placental barrier, including changes associated with known disease pathologies. The group was able to create these placental barrier tissues within a multi-well plate, creating a tissue-on-a-chip assay that may be used in the future to better understand mechanisms of disease which effect the placenta such as gestational hypertension, intrauterine grown restriction, and of course preeclampsia. Further, this assay could be used to screen target compounds for their therapeutic efficacy prior to moving to any in vivo studies in preclinical models of the same disease.
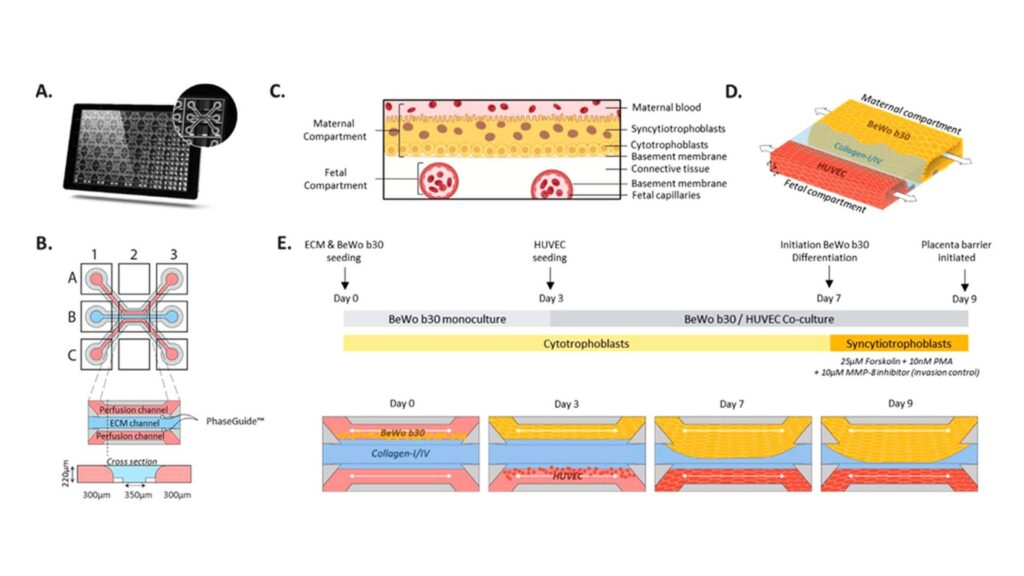
Fig. 1. Placental barrier on-a-chip set-up in the OrganoPlate 3-lane. (A) Bottom view of the OrganoPlate 3-lane, a 384-well plate format containing in its bottom 40 individual microfluidics chips. Zoomed-in image of one microfluid chip. (B) Schematic representation of the chip consisting of three adjacent channels accessible by top (A1), bottom (C1) inlets and top (A3), bottom (C3) outlets wells. Perfusion channels are separated by small ridges (PhaseGuideTM) which enabled the patterning of ECM gel in the middle lane and culture of cells in the perfusion channels in a membrane-free manner. Cultures are observed from the observation window (B2). (C) Schematic of the placental barrier multilayered architecture. The interface is composed of syncytiotrophoblasts (maternal compartment) on top of their basement membrane. A thin connective tissue separates the maternal interface from the fetal capillaries (fetal compartment). (D) 3-dimensional schematic of the placental barrier modeling in the OrganoPlate 3-lane, representing the maternal and fetal compartment in close proximity. Continuous flow through the maternal and fetal compartments is depicted by the arrows. (E) Schematic workflow depicting placental barrier modeling set-up, BeWo b30 differentiation, and controlled invasion over time.
Although not used in the current study, the introduction of 3D bioprinting promises to revolutionize these types of in vitro studies, creating an unprecedented level of physiological relevance. 3D bioprinters, such as the Next Generation Bioprinter for Research (NGB-R) uses biomaterials, cells, and supporting components to print, layer by layer, three-dimensional, functionally viable human tissues, with more complexity then is possible with traditional culture techniques. These fabricated tissues closely mimic in vivo environments, making them exceptional platforms for studying complex diseases like preeclampsia.
Combining the organ/tissue-on-a-chip technology with hypoxia chambers or workstations which allow researchers to very precisely control the amount of available oxygen to the tissues within a study, even allowing those oxygen levels to be modulated and precisely controlled, further allows scientists to study the placental barrier, and its response to changing conditions. The opportunity to manipulate oxygen levels within these printed tissues would provide valuable insights into the hypoxic response mechanisms in preeclampsia.
While studying the effect that changes in environment have on the tissue, not only in the described study above, but in many others, researchers want to be able to monitor, in real-time, the oxygen consumption rate (OCR) of their samples. This can easily be accomplished using the Resipher, which can monitor samples for days or even weeks, in real-time.
Resipher’s potential to measure real-time hypoxia responses in 3D bioprinted tissues could be an invaluable tool for the analysis of preeclampsia pathophysiology. The ability to accurately measure and control oxygen gradients within these tissues will enable a closer examination of the hypoxic response at the cellular and molecular level, providing new insights into the underlying mechanisms driving preeclampsia.
This synergy of innovative tools such as the NGB-R for 3D bioprinting, the wide range of hypoxia workstations, and innovative tools like Resipher represents a transformative shift in the landscape of in vitro studies. This could pave the way for more personalized medicine approaches, informed by a deeper understanding of the molecular mechanisms underpinning preeclampsia, and other pathologies.
Looking ahead, 3D bioprinting may play a crucial role in advancing our knowledge of hypoxia and preeclampsia, and in the broader context, the entire field of gestational disorders. Despite being in its nascent stages, 3D bioprinting has shown immense promise, and combined with tools like Resipher and hypoxia workstations, could change the face of in vitro studies in the near future.
In conclusion, leveraging cutting-edge technologies could catalyze our understanding of complex conditions like preeclampsia. These advances hold the potential to transform the landscape of in vitro studies, empowering researchers to study diseases in a more life-like, controlled environment, and drive the development of effective therapeutic strategies. The scientific community stands on the precipice of a new era in medical research, one that brings us closer than ever to unraveling the intricacies of human health and disease
1. Gwenaëlle Rabussier, Ivan Bünter, Josse Bouwhuis, Camilla Soragni, Torben van Zijp, Chee Ping Ng, Karel Domansky, Leon J. de Windt, Paul Vulto, Colin E. Murdoch, Kristin M. Bircsak, Henriëtte L. Lanz, Healthy and diseased placental barrier on-a-chip models suitable for standardized studies, Acta Biomaterialia, 2023.